Search
- Page Path
- HOME > Search
- Miscellaneous
- Protective Effect of Delta-Like 1 Homolog Against Muscular Atrophy in a Mouse Model
- Ji Young Lee, Minyoung Lee, Dong-Hee Lee, Yong-ho Lee, Byung-Wan Lee, Eun Seok Kang, Bong-Soo Cha
- Endocrinol Metab. 2022;37(4):684-697. Published online August 29, 2022
- DOI: https://doi.org/10.3803/EnM.2022.1446
- 3,199 View
- 129 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
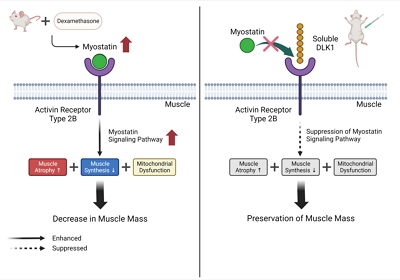
Muscle atrophy is caused by an imbalance between muscle growth and wasting. Delta-like 1 homolog (DLK1), a protein that modulates adipogenesis and muscle development, is a crucial regulator of myogenic programming. Thus, we investigated the effect of exogenous DLK1 on muscular atrophy.
Methods
We used muscular atrophy mouse model induced by dexamethasone (Dex). The mice were randomly divided into three groups: (1) control group, (2) Dex-induced muscle atrophy group, and (3) Dex-induced muscle atrophy group treated with DLK1. The effects of DLK1 were also investigated in an in vitro model using C2C12 myotubes.
Results
Dex-induced muscular atrophy in mice was associated with increased expression of muscle atrophy markers and decreased expression of muscle differentiation markers, while DLK1 treatment attenuated these degenerative changes together with reduced expression of the muscle growth inhibitor, myostatin. In addition, electron microscopy revealed that DLK1 treatment improved mitochondrial dynamics in the Dex-induced atrophy model. In the in vitro model of muscle atrophy, normalized expression of muscle differentiation markers by DLK1 treatment was mitigated by myostatin knockdown, implying that DLK1 attenuates muscle atrophy through the myostatin pathway.
Conclusion
DLK1 treatment inhibited muscular atrophy by suppressing myostatin-driven signaling and improving mitochondrial biogenesis. Thus, DLK1 might be a promising candidate to treat sarcopenia, characterized by muscle atrophy and degeneration.

- Diabetes, Obesity and Metabolism
Big Data Articles (National Health Insurance Service Database) - Improvement in Age at Mortality and Changes in Causes of Death in the Population with Diabetes: An Analysis of Data from the Korean National Health Insurance and Statistical Information Service, 2006 to 2018
- Eugene Han, Sun Ok Song, Hye Soon Kim, Kang Ju Son, Sun Ha Jee, Bong-Soo Cha, Byung-Wan Lee
- Endocrinol Metab. 2022;37(3):466-474. Published online June 29, 2022
- DOI: https://doi.org/10.3803/EnM.2022.1440
- 3,894 View
- 138 Download
- 4 Web of Science
- 4 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
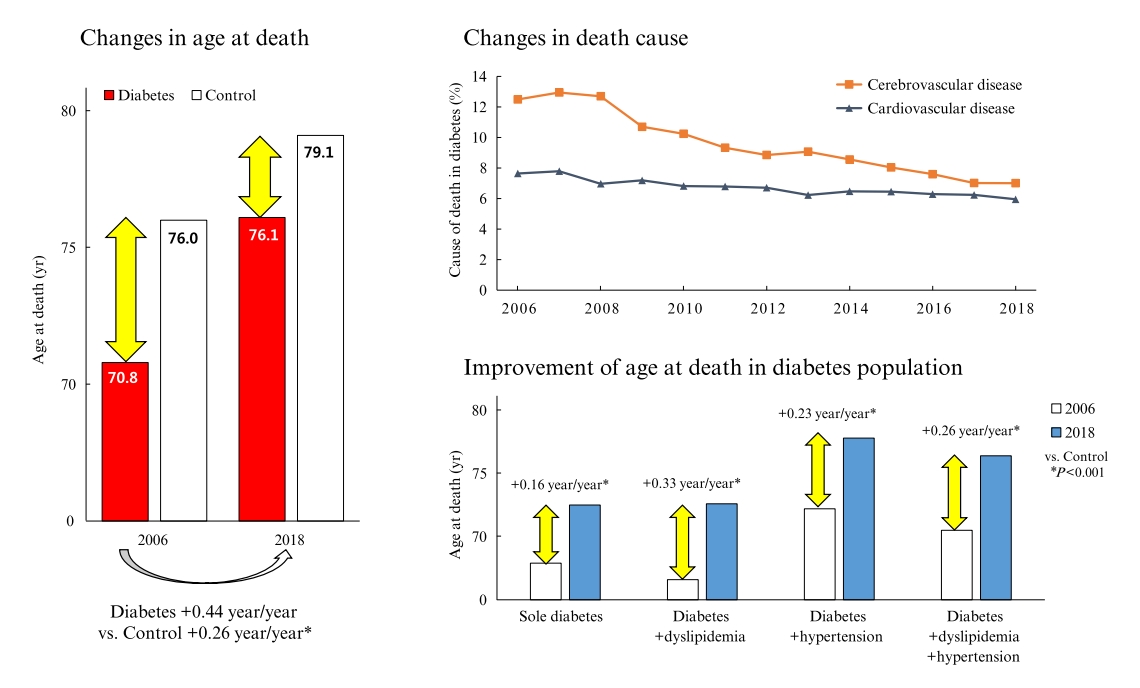
Diabetes is a leading cause of death that is responsible for 1.6 million annual deaths worldwide. However, the life expectancy and age at death of people with diabetes have been a matter of debate.
Methods
The National Health Insurance Service claims database, merged with death records from the National Statistical Information Service in Korea from 2006 to 2018, was analyzed.
Results
In total, 1,432,567 deaths were collected. The overall age at death increased by 0.44 and 0.26 year/year in the diabetes and control populations, respectively. The disparity in the mean age at death between the diabetes and control populations narrowed from 5.2 years in 2006 to 3.0 years in 2018 (p<0.001). In a subgroup analysis according to the presence of comorbid diseases, the number and proportion of deaths remained steady in the group with diabetes only, but steadily increased in the groups with diabetes combined with dyslipidemia and/or hypertension. Compared to the control population, the increase in the mean death age was higher in the population with diabetes. This trend was more prominent in the groups with dyslipidemia and/or hypertension than in the diabetes only group. Deaths from vascular disease and diabetes decreased, whereas deaths from cancer and pneumonia increased. The decline in the proportion of deaths from vascular disease was greater in the diabetes groups with hypertension and/or dyslipidemia than in the control population.
Conclusion
The age at death in the population with diabetes increased more steeply and reached a comparable level to those without diabetes. -
Citations
Citations to this article as recorded by- Analysis of Cause-of-Death Mortality in Children and Young Adults with Diabetes: A Nationwide 10-Year Follow-Up Cohort Study
Iee-Ho Choi, Sang-Woo Yeom, Sun-Young Kim, Jihye You, Jong-Seung Kim, Minsun Kim
Children.2023; 10(2): 358. CrossRef - Age at Mortality in Patients with Type 2 Diabetes Who Underwent Kidney Transplantation: An Analysis of Data from the Korean National Health Insurance and Statistical Information Service, 2006 to 2018
Sun Ok Song, Eugene Han, Kang Ju Son, Bong-Soo Cha, Byung-Wan Lee
Journal of Clinical Medicine.2023; 12(9): 3160. CrossRef - Risk of Cause-Specific Mortality across Glucose Spectrum in Elderly People: A Nationwide Population-Based Cohort Study
Joonyub Lee, Hun-Sung Kim, Kee-Ho Song, Soon Jib Yoo, Kyungdo Han, Seung-Hwan Lee
Endocrinology and Metabolism.2023; 38(5): 525. CrossRef - Long-Term Cumulative Exposure to High γ-Glutamyl Transferase Levels and the Risk of Cardiovascular Disease: A Nationwide Population-Based Cohort Study
Han-Sang Baek, Bongseong Kim, Seung-Hwan Lee, Dong-Jun Lim, Hyuk-Sang Kwon, Sang-Ah Chang, Kyungdo Han, Jae-Seung Yun
Endocrinology and Metabolism.2023; 38(6): 770. CrossRef
- Analysis of Cause-of-Death Mortality in Children and Young Adults with Diabetes: A Nationwide 10-Year Follow-Up Cohort Study

- Clinical Study
- Current Management of Type 2 Diabetes Mellitus in Primary Care Clinics in Korea
- Da Hea Seo, Shinae Kang, Yong-ho Lee, Jung Yoon Ha, Jong Suk Park, Byoung-Wan Lee, Eun Seok Kang, Chul Woo Ahn, Bong-Soo Cha
- Endocrinol Metab. 2019;34(3):282-290. Published online September 26, 2019
- DOI: https://doi.org/10.3803/EnM.2019.34.3.282
- 5,992 View
- 87 Download
- 15 Web of Science
- 15 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub Background This study investigated the overall status of diabetes control and screening for diabetic microvascular complications in patients with type 2 diabetes mellitus attending primary care clinics in Korea.
Methods In this cross-sectional observational study, 191 primary care clinics were randomly selected across Korea from 2015 to 2016. In total, 3,227 subjects were enrolled in the study.
Results The patients followed at the primary care clinics were relatively young, with a mean age of 61.4±11.7 years, and had a relatively short duration of diabetes (mean duration, 7.6±6.5 years). Approximately 14% of subjects had diabetic microvascular complications. However, the patients treated at the primary care clinics had suboptimal control of hemoglobin A1c levels, blood pressure, and serum lipid levels, along with a metabolic target achievement rate of 5.9% according to the Korean Diabetes Association guidelines. The screening rates for diabetic nephropathy, retinopathy, and neuropathy within the past 12 months were 28.4%, 23.3%, and 13.3%, respectively.
Conclusion The overall status of diabetes management, including the frequency of screening for microvascular complications, was suboptimal in the primary care clinics. More efforts should be made and more resources need to be allocated for primary care physicians to promote adequate healthcare delivery, which would result in stricter diabetes control and improved management of diabetic complications.
-
Citations
Citations to this article as recorded by- Risk of Cause-Specific Mortality across Glucose Spectrum in Elderly People: A Nationwide Population-Based Cohort Study
Joonyub Lee, Hun-Sung Kim, Kee-Ho Song, Soon Jib Yoo, Kyungdo Han, Seung-Hwan Lee
Endocrinology and Metabolism.2023; 38(5): 525. CrossRef - Comparison of on-Statin Lipid and Lipoprotein Levels for the Prediction of First Cardiovascular Event in Type 2 Diabetes Mellitus
Ji Yoon Kim, Jimi Choi, Sin Gon Kim, Nam Hoon Kim
Diabetes & Metabolism Journal.2023; 47(6): 837. CrossRef - Effectiveness of quality of care for patients with type 2 diabetes in China: findings from the Shanghai Integration Model (SIM)
Chun Cai, Yuexing Liu, Yanyun Li, Yan Shi, Haidong Zou, Yuqian Bao, Yun Shen, Xin Cui, Chen Fu, Weiping Jia
Frontiers of Medicine.2022; 16(1): 126. CrossRef - Comparison of Health Outcomes by Care Provider Type for Newly Diagnosed Mild Type 2 Diabetes Patients in South Korea: A Retrospective Cohort Study
Hee-Chung Kang, Jae-Seok Hong
Healthcare.2022; 10(2): 334. CrossRef - Management Status of Patients with Type 2 Diabetes Mellitus at General Hospitals in Korea: A 5-Year Follow-Up Study
Jin Hee Jung, Jung Hwa Lee, Hyang Mi Jang, Young Na, Hee Sun Choi, Yeon Hee Lee, Yang Gyo Kang, Na Rae Kim, Jeong Rim Lee, Bok Rye Song, Kang Hee Sim
The Journal of Korean Diabetes.2022; 23(1): 64. CrossRef - Type 2 Diabetes Mellitus with Early Dry Skin Disorder: A Comparison Study Between Primary and Tertiary Care in Indonesia
Lili Legiawati, Kusmarinah Bramono, Wresti Indriatmi, Em Yunir, Aditya Indra Pratama
Current Diabetes Reviews.2022;[Epub] CrossRef - Long-Term Changes in HbA1c According to Blood Glucose Control Status During the First 3 Months After Visiting a Tertiary University Hospital
Hyunah Kim, Da Young Jung, Seung-Hwan Lee, Jae-Hyoung Cho, Hyeon Woo Yim, Hun-Sung Kim
Journal of Korean Medical Science.2022;[Epub] CrossRef - Differences in health behavior and nutrient intake status between diabetes-aware and unaware Korean adults based on the Korea national health and nutrition examination survey 2016–18 data: A cross-sectional study
Anshul Sharma, Chen Lulu, Kee-Ho Song, Hae-Jeung Lee
Frontiers in Public Health.2022;[Epub] CrossRef - Effects of Diabetes Quality Assessment on Diabetes Management Behaviors Based on a Nationwide Survey
Chang Kyun Choi, Jungho Yang, Ji-An Jeong, Min-Ho Shin
International Journal of Environmental Research and Public Health.2022; 19(23): 15781. CrossRef - The Impact of the Indonesian Chronic Disease Management Program (PROLANIS) on Metabolic Control and Renal Function of Type 2 Diabetes Mellitus Patients in Primary Care Setting
Firas Farisi Alkaff, Fauzan Illavi, Sovia Salamah, Wiwit Setiyawati, Ristra Ramadhani, Elly Purwantini, Dicky L. Tahapary
Journal of Primary Care & Community Health.2021; 12: 215013272098440. CrossRef - Questionnaire-based Survey of Demographic and Clinical Characteristics, Health Behaviors, and Mental Health of Young Korean Adults with Early-Onset Diabetes
Ji In Park, Hyunjeong Baek, Sang-Wook Kim, Ji Yun Jeong, Kee-Ho Song, Ji Hee Yu, Il Sung Nam-Goong, Eun-Hee Cho
Journal of Korean Medical Science.2021;[Epub] CrossRef - Sodium–Glucose Cotransporter 2 Inhibitors and Risk of Retinal Vein Occlusion Among Patients With Type 2 Diabetes: A Propensity Score–Matched Cohort Study
Min-Kyung Lee, Bongsung Kim, Kyungdo Han, Jae-Hyuk Lee, Minhee Kim, Mee Kyoung Kim, Ki-Hyun Baek, Ki-Ho Song, Hyuk-Sang Kwon, Young-Jung Roh
Diabetes Care.2021; 44(10): 2419. CrossRef - Challenges in the Management of Diabetes in Primary Care
Yeon Kyung Lee
The Journal of Korean Diabetes.2020; 21(3): 161. CrossRef - Does Diabetes Increase the Risk of Contracting COVID-19? A Population-Based Study in Korea
Sung-Youn Chun, Dong Wook Kim, Sang Ah Lee, Su Jung Lee, Jung Hyun Chang, Yoon Jung Choi, Seong Woo Kim, Sun Ok Song
Diabetes & Metabolism Journal.2020; 44(6): 897. CrossRef - Comprehensive Efforts Are Needed to Improve the Quality of Primary Diabetes Care in Korea
Chan-Hee Jung
Endocrinology and Metabolism.2019; 34(3): 265. CrossRef
- Risk of Cause-Specific Mortality across Glucose Spectrum in Elderly People: A Nationwide Population-Based Cohort Study

- Obesity and Metabolism
- Comparison of the Effects of Ezetimibe-Statin Combination Therapy on Major Adverse Cardiovascular Events in Patients with and without Diabetes: A Meta-Analysis
- Namki Hong, Yong-ho Lee, Kenichi Tsujita, Jorge A. Gonzalez, Christopher M. Kramer, Tomas Kovarnik, George N. Kouvelos, Hiromichi Suzuki, Kyungdo Han, Chan Joo Lee, Sung Ha Park, Byung-Wan Lee, Bong-Soo Cha, Eun Seok Kang
- Endocrinol Metab. 2018;33(2):219-227. Published online May 4, 2018
- DOI: https://doi.org/10.3803/EnM.2018.33.2.219
- 5,724 View
- 125 Download
- 18 Web of Science
- 17 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub Background Ezetimibe-statin combination therapy has been found to reduce low density lipoprotein cholesterol levels and the risk of major adverse cardiovascular events (MACEs) in large trials. We sought to examine the differential effect of ezetimibe on MACEs when added to statins according to the presence of diabetes.
Methods Randomized clinical trials with a sample size of at least 50 participants and at least 24 weeks of follow-up that compared ezetimibe-statin combination therapy with a statin- or placebo-controlled arm and reported at least one MACE, stratified by diabetes status, were included in the meta-analysis and meta-regression.
Results A total of seven trials with 28,191 enrolled patients (mean age, 63.6 years; 75.1% men; 7,298 with diabetes [25.9%]; mean follow-up, 5 years) were analysed. MACEs stratified by diabetes were obtained from the published data (two trials) or through direct contact (five trials). No significant heterogeneity was observed among studies (
I 2=14.7%,P =0.293). Ezetimibe was associated with a greater reduction of MACE risk in subjects with diabetes than in those without diabetes (pooled relative risk, 0.84 vs. 0.93;P heterogeneity=0.012). In the meta-regression analysis, the presence of diabetes was associated with a greater reduction of MACE risk when ezetimibe was added to statins (β=0.87,P =0.038).Conclusion Ezetimibe-statin combination therapy was associated with greater cardiovascular benefits in patients with diabetes than in those without diabetes. Our findings suggest that ezetimibe-statin combination therapy might be a useful strategy in patients with diabetes at a residual risk of MACEs.
-
Citations
Citations to this article as recorded by- Multifunctional nanoparticle-mediated combining therapy for human diseases
Xiaotong Li, Xiuju Peng, Makhloufi Zoulikha, George Frimpong Boafo, Kosheli Thapa Magar, Yanmin Ju, Wei He
Signal Transduction and Targeted Therapy.2024;[Epub] CrossRef - Comparative Efficacy of Rosuvastatin Monotherapy and Rosuvastatin/Ezetimibe Combination Therapy on Insulin Sensitivity and Vascular Inflammatory Response in Patients with Type 2 Diabetes Mellitus
Ji Hye Han, Kyong Hye Joung, Jun Choul Lee, Ok Soon Kim, Sorim Choung, Ji Min Kim, Yea Eun Kang, Hyon-Seung Yi, Ju Hee Lee, Bon Jeong Ku, Hyun Jin Kim
Diabetes & Metabolism Journal.2024; 48(1): 112. CrossRef - Combining Ezetimibe and Rosuvastatin: Impacts on Insulin Sensitivity and Vascular Inflammation in Patients with Type 2 Diabetes Mellitus
Eun Roh
Diabetes & Metabolism Journal.2024; 48(1): 55. CrossRef - Efficacy and Safety of Pitavastatin/Ezetimibe Fixed-Dose Combination vs. Pitavastatin: Phase III, Double-Blind, Randomized Controlled Trial
Kenichi Tsujita, Koutaro Yokote, Junya Ako, Ryohei Tanigawa, Sachiko Tajima, Hideki Suganami
Journal of Atherosclerosis and Thrombosis.2023; 30(11): 1580. CrossRef - 2023 Clinical Practice Guidelines for Diabetes Mellitus of the Korean Diabetes Association
Jong Han Choi, Kyung Ae Lee, Joon Ho Moon, Suk Chon, Dae Jung Kim, Hyun Jin Kim, Nan Hee Kim, Ji A Seo, Mee Kyoung Kim, Jeong Hyun Lim, YoonJu Song, Ye Seul Yang, Jae Hyeon Kim, You-Bin Lee, Junghyun Noh, Kyu Yeon Hur, Jong Suk Park, Sang Youl Rhee, Hae J
Diabetes & Metabolism Journal.2023; 47(5): 575. CrossRef - Ezetimibe combination therapy with statin for non-alcoholic fatty liver disease: an open-label randomized controlled trial (ESSENTIAL study)
Yongin Cho, Hyungjin Rhee, Young-eun Kim, Minyoung Lee, Byung-Wan Lee, Eun Seok Kang, Bong-Soo Cha, Jin-Young Choi, Yong-ho Lee
BMC Medicine.2022;[Epub] CrossRef - RSSDI consensus recommendations for dyslipidemia management in diabetes mellitus
Banshi Saboo, Sanjay Agarwal, Brij Mohan Makkar, Rajeev Chawla, Sujoy Ghosh, Vijay Viswanathan, Sunil Gupta, Ch. Vasanth Kumar, Anuj Maheshwari, L. Sreenivasamurthy, Rakesh Kumar Sahay, Sanjay Reddy, Shalini Jaggi, Jugal Kishor Sharma, Vijay Panikar, Anan
International Journal of Diabetes in Developing Countries.2022; 42(1): 3. CrossRef - Ezetimibe and diabetes mellitus:a new strategy for lowering cholesterol
V.A. Serhiyenko, A.A. Serhiyenko
INTERNATIONAL JOURNAL OF ENDOCRINOLOGY (Ukraine).2022; 18(5): 302. CrossRef - 2021 Clinical Practice Guidelines for Diabetes Mellitus of the Korean Diabetes Association
Kyu Yeon Hur, Min Kyong Moon, Jong Suk Park, Soo-Kyung Kim, Seung-Hwan Lee, Jae-Seung Yun, Jong Ha Baek, Junghyun Noh, Byung-Wan Lee, Tae Jung Oh, Suk Chon, Ye Seul Yang, Jang Won Son, Jong Han Choi, Kee Ho Song, Nam Hoon Kim, Sang Yong Kim, Jin Wha Kim,
Diabetes & Metabolism Journal.2021; 45(4): 461. CrossRef - Combination of Statin and Ezetimibe versus Statin Monotherapy on Cardiovascular Disease and Type 2 Diabetes Incidence among Adults with Impaired Fasting Glucose: a Propensity-Matched Nationwide Cohort Study
You-Bin Lee, Bongsung Kim, Kyungdo Han, Jung A Kim, Eun Roh, So-hyeon Hong, Kyung Mook Choi, Sei Hyun Baik, Hye Jin Yoo
Journal of Lipid and Atherosclerosis.2021; 10(3): 303. CrossRef - PCSK9 inhibitors and cardiovascular outcomes
Daniel Steffens, Peter Bramlage, Céline Scheeff, Mario Kasner, Adel Hassanein, Julian Friebel, Ursula Rauch-Kröhnert
Expert Opinion on Biological Therapy.2020; 20(1): 35. CrossRef - Comparison of Renal Effects of Ezetimibe–Statin Combination versus Statin Monotherapy: A Propensity-Score-Matched Analysis
Jaehyun Bae, Namki Hong, Byung-Wan Lee, Eun Seok Kang, Bong-Soo Cha, Yong-ho Lee
Journal of Clinical Medicine.2020; 9(3): 798. CrossRef - Effect of Ezetimibe on Glucose Metabolism and Inflammatory Markers in Adipose Tissue
Yongin Cho, Ryeong-Hyeon Kim, Hyunki Park, Hye Jin Wang, Hyangkyu Lee, Eun Seok Kang
Biomedicines.2020; 8(11): 512. CrossRef - Future perspectives of the pharmacological management of diabetic dyslipidemia
Angelo Maria Patti, Rosaria Vincenza Giglio, Nikolaos Papanas, Manfredi Rizzo, Ali A. Rizvi
Expert Review of Clinical Pharmacology.2019; 12(2): 129. CrossRef - Diabetes and Vascular Disease: Is It All About Glycemia?
Alessandra Vecchié, Fabrizio Montecucco, Federico Carbone, Franco Dallegri, Aldo Bonaventura
Current Pharmaceutical Design.2019; 25(29): 3112. CrossRef - Triennial Report ofEndocrinology and Metabolism, 2015 to 2017
Eun-Jung Rhee, Hey Yeon Jang, Won-Young Lee
Endocrinology and Metabolism.2018; 33(2): 195. CrossRef - Guidelines, Clinical Evidence, and Real-Life Practice: How to Find Your Way in Managing Hypercholesterolaemia
Janet Fricker
EMJ Cardiology.2018; : 38. CrossRef
- Multifunctional nanoparticle-mediated combining therapy for human diseases

- Miscellaneous
- Effects of Serum Albumin, Calcium Levels, Cancer Stage and Performance Status on Weight Loss in Parathyroid Hormone-Related Peptide Positive or Negative Patients with Cancer
- Ji-Yeon Lee, Namki Hong, Hye Ryun Kim, Byung Wan Lee, Eun Seok Kang, Bong-Soo Cha, Yong-ho Lee
- Endocrinol Metab. 2018;33(1):97-104. Published online March 21, 2018
- DOI: https://doi.org/10.3803/EnM.2018.33.1.97
- 4,435 View
- 45 Download
- 8 Web of Science
- 5 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub Background A recent animal study showed that parathyroid hormone-related peptide (PTHrP) is associated with cancer cachexia by promoting adipose tissue browning, and we previously demonstrated that PTHrP predicts weight loss (WL) in patients with cancer. In this study, we investigated whether prediction of WL by PTHrP is influenced by clinical factors such as serum albumin, corrected calcium levels, cancer stage, and performance status (PS).
Methods A cohort of 219 patients with cancer whose PTHrP level was measured was enrolled and followed for body weight (BW) changes. Subjects were divided into two groups by serum albumin (cutoff value, 3.7 g/dL), corrected calcium (cutoff value, 10.5 mg/dL), cancer stage (stage 1 to 3 or 4), or PS (Eastern Cooperative Oncology Group 0 to 1 or 2 to 4), respectively. Clinically significant WL was defined as either percent of BW change (% BW) <−5% or % BW <−2% plus body mass index (BMI) <20 kg/m2.
Results After a median follow-up of 327 days, 74 patients (33.8%) experienced clinically significant WL. A positive PTHrP level was associated with a 2-fold increased risk of WL after adjusting for age, baseline BMI, serum albumin, corrected calcium level, cancer stage, and PS. The effect of PTHrP on WL remained significant in patients with low serum albumin, stage 4 cancer, and good PS. Regardless of calcium level, the effect of PTHrP on WL was maintained, although there was an additive effect of higher calcium and PTHrP levels.
Conclusion Early recognition of patients with advanced cancer who are PTHrP positive with hypercalcemia or hypoalbuminemia is needed for their clinical management.
-
Citations
Citations to this article as recorded by- Can Patients with Electrolyte Disturbances Be Safely and Effectively Treated in a Hospital-at-Home, Telemedicine-Controlled Environment? A Retrospective Analysis of 267 Patients
Cohn May, Gueron Or, Segal Gad, Zubli Daniel, Hakim Hila, Fizdel Boris, Liber Pninit, Amir Hadar, Barkai Galia
Journal of Clinical Medicine.2024; 13(5): 1409. CrossRef - Parathyroid hormone related protein (PTHrP) in patients with pancreatic carcinoma and overt signs of disease progression and host tissue wasting
Britt-Marie Iresjö, Serkan Kir, Kent Lundholm
Translational Oncology.2023; 36: 101752. CrossRef - Development and Characterization of a Cancer Cachexia Rat Model Transplanted with Cells of the Rat Lung Adenocarcinoma Cell Line Sato Lung Cancer (SLC)
Eiji Kasumi, Miku Chiba, Yoshie Kuzumaki, Hiroyuki Kuzuoka, Norifumi Sato, Banyu Takahashi
Biomedicines.2023; 11(10): 2824. CrossRef - Inhibition of epidermal growth factor receptor suppresses parathyroid hormone‐related protein expression in tumours and ameliorates cancer‐associated cachexia
Bahar Zehra Camurdanoglu Weber, Samet Agca, Aylin Domaniku, Sevval Nur Bilgic, Dilsad H. Arabaci, Serkan Kir
Journal of Cachexia, Sarcopenia and Muscle.2022; 13(3): 1582. CrossRef - Metabolic Reprogramming in Adipose Tissue During Cancer Cachexia
Bahar Zehra Camurdanoglu Weber, Dilsad H. Arabaci, Serkan Kir
Frontiers in Oncology.2022;[Epub] CrossRef
- Can Patients with Electrolyte Disturbances Be Safely and Effectively Treated in a Hospital-at-Home, Telemedicine-Controlled Environment? A Retrospective Analysis of 267 Patients

- Comparison between Atorvastatin and Rosuvastatin in Renal Function Decline among Patients with Diabetes
- Eugene Han, Gyuri Kim, Ji-Yeon Lee, Yong-ho Lee, Beom Seok Kim, Byung-Wan Lee, Bong-Soo Cha, Eun Seok Kang
- Endocrinol Metab. 2017;32(2):274-280. Published online June 23, 2017
- DOI: https://doi.org/10.3803/EnM.2017.32.2.274
- 5,243 View
- 175 Download
- 8 Web of Science
- 8 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Although the beneficial effects of statin treatment in dyslipidemia and atherosclerosis have been well studied, there is limited information regarding the renal effects of statins in diabetic nephropathy. We aimed to investigate whether, and which, statins affected renal function in Asian patients with diabetes.
Methods We enrolled 484 patients with diabetes who received statin treatment for more than 12 months. We included patients treated with moderate-intensity dose statin treatment (atorvastatin 10 to 20 mg/day or rosuvastatin 5 to 10 mg/day). The primary outcome was a change in estimated glomerular filtration rate (eGFR) during the 12-month statin treatment, and rapid renal decline was defined as a >3% reduction in eGFR in a 1-year period.
Results In both statin treatment groups, patients showed improved serum lipid levels and significantly reduced eGFRs (from 80.3 to 78.8 mL/min/1.73 m2 for atorvastatin [
P =0.012], from 79.1 to 76.1 mL/min/1.73 m2 for rosuvastatin [P =0.001]). A more rapid eGFR decline was observed in the rosuvastatin group than in the atorvastatin group (48.7% vs. 38.6%,P =0.029). Multiple logistic regression analyses demonstrated more rapid renal function loss in the rosuvastatin group than in the atorvastatin group after adjustment for other confounding factors (odds ratio, 1.60; 95% confidence interval, 1.06 to 2.42).Conclusion These results suggest that a moderate-intensity dose of atorvastatin has fewer detrimental effects on renal function than that of rosuvastatin.
-
Citations
Citations to this article as recorded by- Efficacy and safety of combination therapy with telmisartan, rosuvastatin, and ezetimibe in patients with dyslipidemia and hypertension: A randomized, double‐blind, multicenter, therapeutic confirmatory, phase III clinical trial
Chan Joo Lee, Woong Chol Kang, Sang Hyun Ihm, Il Suk Sohn, Jong Shin Woo, Jin Won Kim, Soon Jun Hong, Jung Hyun Choi, Jung‐Won Suh, Jae‐Bin Seo, Joon‐Hyung Doh, Jung‐Woo Son, Jae‐Hyeong Park, Ju‐Hee Lee, Young Joon Hong, Jung Ho Heo, Jinho Shin, Seok‐Min
The Journal of Clinical Hypertension.2024; 26(3): 262. CrossRef - Anti-hyperglycemic, anti-hyperlipidemic, and anti-inflammatory effect of the drug Guggulutiktaka ghrita on high-fat diet-induced obese rats
Samreen M. Sheik, Pugazhandhi Bakthavatchalam, Revathi P. Shenoy, Basavaraj S. Hadapad, Deepak Nayak M, Monalisa Biswas, Varashree Bolar Suryakanth
Journal of Ayurveda and Integrative Medicine.2022; 13(3): 100583. CrossRef - The challenge of reducing residual cardiovascular risk in patients with chronic kidney disease
Stefan Mark Nidorf
European Heart Journal.2022; 43(46): 4845. CrossRef - Diabetic Kidney Disease in Older People with Type 2 Diabetes Mellitus: Improving Prevention and Treatment Options
Ahmed H. Abdelhafiz
Drugs & Aging.2020; 37(8): 567. CrossRef - Intracellular Mechanism of Rosuvastatin-Induced Decrease in Mature hERG Protein Expression on Membrane
Pan-Feng Feng, Bo Zhang, Lei Zhao, Qing Fang, Yan Liu, Jun-Nan Wang, Xue-Qi Xu, Hui Xue, Yang Li, Cai-Chuan Yan, Xin Zhao, Bao-Xin Li
Molecular Pharmaceutics.2019; 16(4): 1477. CrossRef - The problem of safety of lipid-lowering therapy
M V. Zykov
Kardiologiia.2019; 59(5S): 13. CrossRef - Regional evidence and international recommendations to guide lipid management in Asian patients with type 2 diabetes with special reference to renal dysfunction
Titus WL Lau, Kevin E.K. Tan, Jason C.J. Choo, Tsun‐Gun Ng, Subramaniam Tavintharan, Juliana C.N. Chan
Journal of Diabetes.2018; 10(3): 200. CrossRef - Lipids: a personal view of the past decade
Niki Katsiki, Dimitri P Mikhailidis
Hormones.2018; 17(4): 461. CrossRef
- Efficacy and safety of combination therapy with telmisartan, rosuvastatin, and ezetimibe in patients with dyslipidemia and hypertension: A randomized, double‐blind, multicenter, therapeutic confirmatory, phase III clinical trial

- Obesity and Metabolism
- Optimal Candidates for the Switch from Glimepiride to Sitagliptin to Reduce Hypoglycemia in Patients with Type 2 Diabetes Mellitus
- Hyun Min Kim, Jung Soo Lim, Byung-Wan Lee, Eun-Seok Kang, Hyun Chul Lee, Bong-Soo Cha
- Endocrinol Metab. 2015;30(1):84-91. Published online March 27, 2015
- DOI: https://doi.org/10.3803/EnM.2015.30.1.84
- 5,858 View
- 98 Download
- 12 Web of Science
- 11 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Sitagliptin is a novel antidiabetic agent with a low risk for hypoglycemia. We investigated the efficacy and safety of sitagliptin when patients switched from a sulfonylurea to sitagliptin and identified good candidates for the switch.
Methods Sixty-one patients with type 2 diabetes switched from glimepiride with metformin to sitagliptin with metformin due to clinical hypoglycemia. Serum glycated hemoglobin (HbA1c), fasting plasma glucose (FPG), and 2-hour postprandial plasma glucose (2h-PPG) before and 12 and 24 weeks after the drug switch were checked.
Results HbA1c and FPG levels did not change 12 or 24 weeks after the switch; however, the 2h-PPG level decreased from 218.0±67.5 mg/dL at baseline to 197.1±69.9 mg/dL at 12 weeks and 192.3±67.4 mg/dL at 24 weeks after switching drugs (
P =0.045,P =0.018, respectively). All but one patient no longer experienced hypoglycemia after discontinuing glimepiride. In a multivariate logistic regression analysis, a high homeostasis model assessment of insulin resistance and low baseline HbA1c level were independent predictors of an HbA1c ≤7% after switching to sitagliptin.Conclusion Glycemic control was not aggravated in patients 24 weeks after the drug switch, and symptomatic hypoglycemia decreased significantly. Patients with dominant insulin resistance may be good candidates for switching from a sulfonylurea to sitagliptin to reduce hypoglycemia.
-
Citations
Citations to this article as recorded by- Application of Machine Learning Methods for the Development of Antidiabetic
Drugs
Juanjuan Zhao, Pengcheng Xu, Xiujuan Liu, Xiaobo Ji, Minjie Li, Dev Sooranna, Xiaosheng Qu, Wencong Lu, Bing Niu
Current Pharmaceutical Design.2022; 28(4): 260. CrossRef - Efficacy and safety of dorzagliatin for type 2 diabetes mellitus: A meta-analysis and trial sequential analysis
Yunfeng Yu, Xingyu Yang, Keke Tong, Shuang Yin, Gang Hu, Fei Zhang, Pengfei Jiang, Manli Zhou, Weixiong Jian
Frontiers in Cardiovascular Medicine.2022;[Epub] CrossRef - Factors associated with switching from sulphonylureas to dipeptidyl peptidase 4 inhibitors among patients with type 2 diabetes in the United States
Xi Tan, Lingfeng Yang, Kamlesh Khunti, Ruya Zhang, Ye Zhang, Swapnil Rajpathak, Miao Yu
Diabetes, Obesity and Metabolism.2021; 23(10): 2251. CrossRef - Increased risk of adverse cardiovascular events by strict glycemic control after percutaneous coronary intervention (HbA1c < 6.5% at 2 years) in type 2 diabetes mellitus combined with acute coronary syndrome: a 5-years follow-up study
Tiangui Yang, Peng Fu, Jie Chen, Xi Fu, Changlu Xu, Xiaoxia Liu, Tiesheng Niu
Current Medical Research and Opinion.2021; 37(9): 1517. CrossRef - Clinical analysis of pre-existing diabetes mellitus and dipeptidyl peptidase-4 inhibitors in patients with remitting seronegative symmetrical synovitis and pitting edema syndrome
Yoshiro Horai, Tomoki Origuchi, Nozomi Iwanaga, Junichi Tokumitsu, Toshiyuki Ikeoka, Genpei Kuriya, Yasumori Izumi, Atsushi Kawakami
Modern Rheumatology.2020; 30(4): 703. CrossRef - The efficacy and safety of dipeptidyl peptidase-4 inhibitors for type 2 diabetes: a Bayesian network meta-analysis of 58 randomized controlled trials
Juan Ling, Peng Cheng, Long Ge, Ding-hua Zhang, An-chen Shi, Jin-hui Tian, Ya-jing Chen, Xiu-xia Li, Jing-yun Zhang, Ke-hu Yang
Acta Diabetologica.2019; 56(3): 249. CrossRef - Satisfaction and efficacy of switching from daily dipeptidyl peptidase-4 inhibitors to weekly trelagliptin in patients with type 2 diabetes—Randomized controlled study—
Mayuko Oita, Hideaki Miyoshi, Kota Ono, Akinobu Nakamura, Kyu Yong Cho, Hiroshi Nomoto, Kohei Yamamoto, Kazuno Omori, Naoki Manda, Yoshio Kurihara, Shin Aoki, Tatsuya Atsumi
Endocrine Journal.2018; 65(2): 141. CrossRef - Efficacy and safety of evogliptin versus sitagliptin as add on to metformin alone in a combined russian-korean population. Evo-combi trial
Alina Y. Babenko, Anna A. Mosikian, Igor E. Makarenko, Victoriya V. Leusheva, Evgeny V. Shlyakhto
Diabetes mellitus.2018; 21(4): 241. CrossRef - Effectiveness prediction of Evogliptin treatment in type 2 diabetes mellitus in russian-korean population
Anna A. Mosikian, Alina Y. Babenko, Yulia A. Sevastyanova, Roman V. Drai, Evgenij V. Shlyakhto
Diabetes mellitus.2018; 21(5): 333. CrossRef - Comprehensive analysis of the Co-structures of dipeptidyl peptidase IV and its inhibitor
Hiroyuki Nojima, Kazuhiko Kanou, Genki Terashi, Mayuko Takeda-Shitaka, Gaku Inoue, Koichiro Atsuda, Chihiro Itoh, Chie Iguchi, Hajime Matsubara
BMC Structural Biology.2016;[Epub] CrossRef - A genetic variant in GLP1R is associated with response to DPP-4 inhibitors in patients with type 2 diabetes
Eugene Han, Hye Sun Park, Obin Kwon, Eun Yeong Choe, Hye Jin Wang, Yong-ho Lee, Sang-Hak Lee, Chul Hoon Kim, Lee-Kyung Kim, Soo Heon Kwak, Kyong Soo Park, Chul Sik Kim, Eun Seok Kang
Medicine.2016; 95(44): e5155. CrossRef
- Application of Machine Learning Methods for the Development of Antidiabetic
Drugs


 KES
KES

 First
First Prev
Prev



